- Home
- Features
- Business
- Active
- Sports
- Shop
Top Insights

In July 2018, FDA released the Biosimilars Action Plan (BAP), which outlined FDA’s approach for expanding access to biosimilars for the American public. The plan focused on 4 key areas:
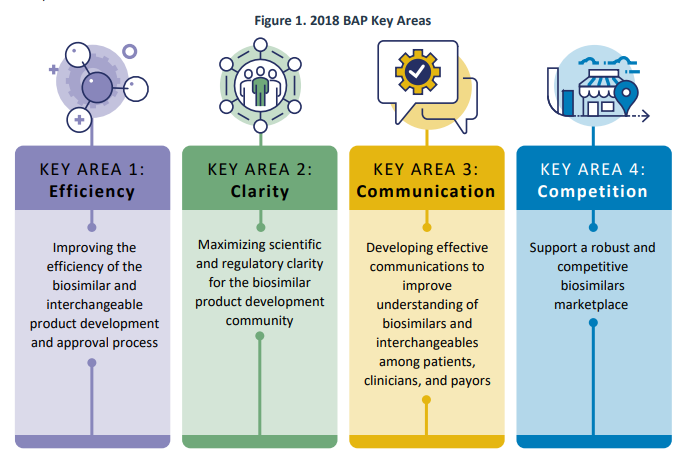
A recent FDA report reviews some of their accomplishments since then. Most of these efforts revolve around guidance documents, additional staff, education products and websites, public hearings and regulations (i.e., proposed/final rules). FDA also added new data resources including publishing a modernized version of the Purple Book in February 2020. FDA also collaborated with other agencies such as FTC, and produced a joint statement and held a workshop in March 2020, entitled: “Public Workshop: FDA/FTC Workshop on a Competitive Marketplace for Biosimilars.” Some other key actions are listed below.

The full report is here.
Recent Posts
Categories
Related Articles
Carlyle and SK Capital Partners are buying beleaguered gene therapy biotech Bluebird...
ByglobalreutersFebruary 21, 2025Hospitals are adopting AI technology more than ever before, but they still...
ByglobalreutersFebruary 21, 2025This fact sheet provides an overview of the history of the Kemp-Kasten...
ByglobalreutersFebruary 21, 2025





Leave a comment