- Home
- Features
- Business
- Active
- Sports
- Shop
Top Insights
© Copyright 2022 Jellywp. All rights reserved powered by Jellywp.com
Home
Health
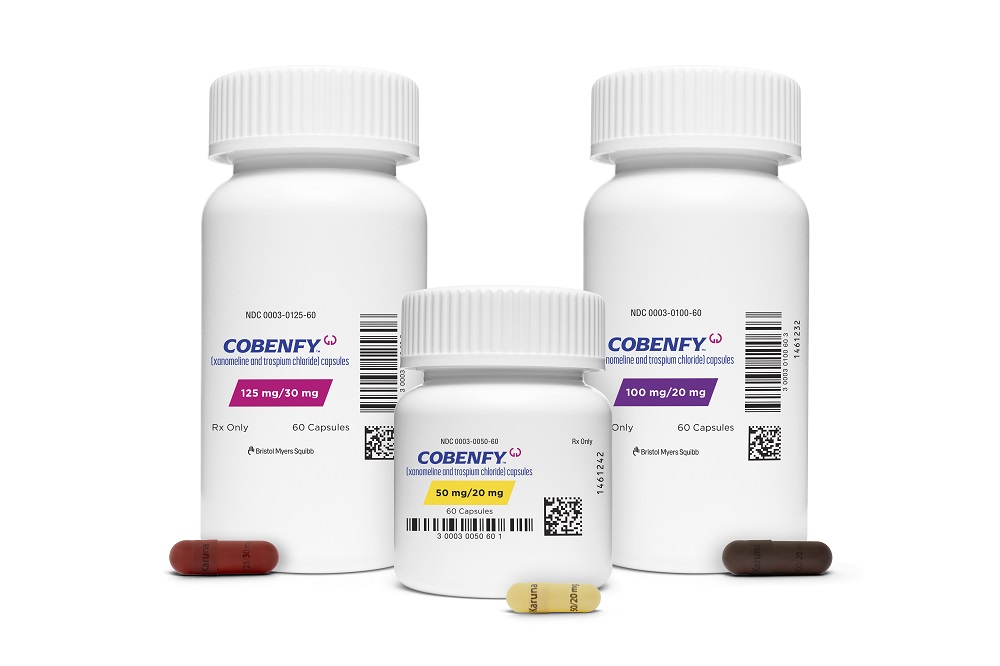
FDA Approval of Bristol Myers Drug Makes It the First Novel Schizophrenia Med in Decades
Health FDA Approval of Bristol Myers Drug Makes It the First Novel Schizophrenia Med in Decades

Bristol Myers Squibb’s Cobenfy treats schizophrenia by going after a different target than currently available antipsychotic drugs, which is intended to offer better efficacy and safety. The drug came from the pharma giant’s $14 billion acquisition of Karuna Therapeutics.
The post FDA Approval of Bristol Myers Drug Makes It the First Novel Schizophrenia Med in Decades appeared first on MedCity News.
Recent Posts
Categories
Related Articles
Carlyle and SK Capital Partners are buying beleaguered gene therapy biotech Bluebird...
ByglobalreutersFebruary 21, 2025Hospitals are adopting AI technology more than ever before, but they still...
ByglobalreutersFebruary 21, 2025This fact sheet provides an overview of the history of the Kemp-Kasten...
ByglobalreutersFebruary 21, 2025








Leave a comment